AUSTEDO XR should be taken:
Once daily
With water
Whole (do not
crush or break)
With or without food
Voucher and NEW Patient Copay Assistance information is now available here
Once-daily AUSTEDO XR (deutetrabenazine) extended-release tablets is a titrated medication, which means your doctor will adjust your dose up or down until you find the dose that provides effective movement reduction while ensuring the medicine is tolerated.

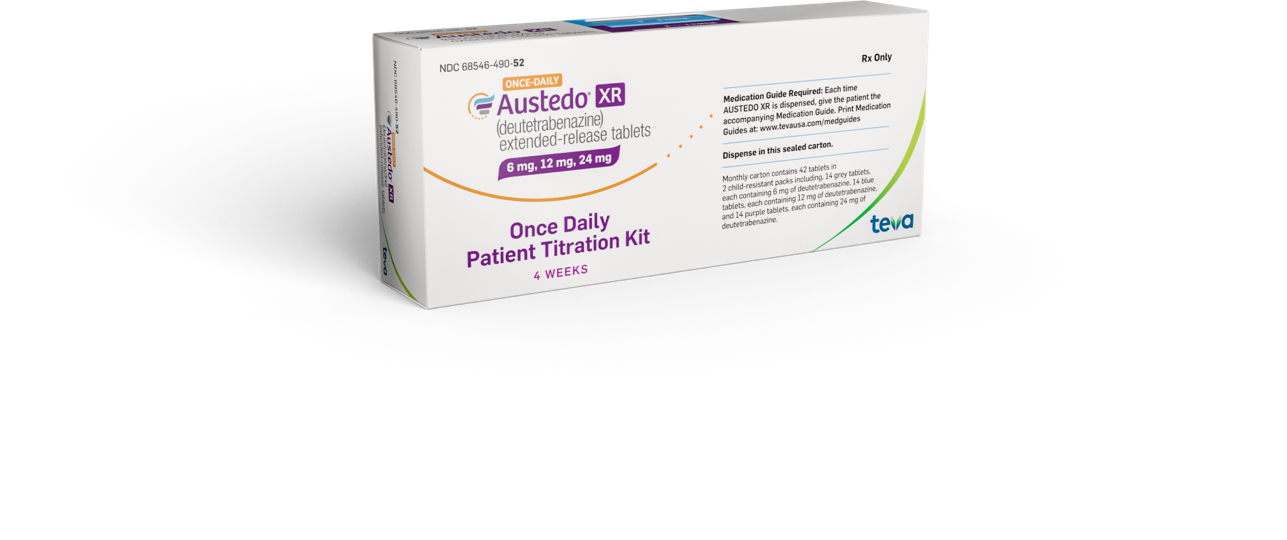
Available as a sample or with a prescription from your doctor
Sample Titration Kit
Given to you at your doctor’s office
or
Prescription Titration Kit
Prescription given to you to be filled at your pharmacy. Participating pharmacies will apply a 30-day Free Trial Voucher* to your prescription so it is no cost to you.
*The 30-Day Free Trial Voucher is only for new patients. Voucher is good for up to 4 weeks of titration or 30 days of medication.
Packaging not to scale.
This is the dosing schedule for the Titration Kit. Upon completion of the kit, your doctor will write you a prescription for AUSTEDO XR and determine what daily dose is right for you.
Titration Schedule

Once-Daily Dosing (mg)
Dosing Totals
Week 1


12 mg
Week 2



18 mg
Week 3


24 mg
Week 4



30 mg
Titration
Schedule
Once-daily
Dosing (mg)
Dosing
Totals
Week 1

12 mg
Week 2


18 mg
Week 3

24 mg
Week 4


30 mg
AUSTEDO XR is available in 6 mg, 12 mg, and 24 mg extended-release tablets and can be combined up to a daily maximum strength of 48 mg. In one clinical study, the average dose of AUSTEDO® (deutetrabenazine) tablets after treatment was ~38 mg daily.*
*Once-daily AUSTEDO XR contains the same active ingredient as twice-daily AUSTEDO. Data on this page is based on twice-daily dosing.1 Pills are for illustration only and not actual size or likeness.
Reaching your maintenance dose1

Once you reach a dose that provides effective movement reduction and is tolerated, that will become your maintenance dose—the dose you stay on moving forward.
It’s important to take AUSTEDO XR exactly as prescribed by your doctor. If you experience a change in symptoms, share these details with your doctor as they may decide to adjust your dose.
Once daily
With water
Whole (do not
crush or break)
With or without food
Twice daily
With water
Whole (do not
crush or break)
With food
If you cannot swallow AUSTEDO XR/AUSTEDO tablets whole, tell your healthcare provider. You may need a different medication.
Use these tips to help ensure you take AUSTEDO XR as prescribed:
For additional information about how to take AUSTEDO XR, please read the Medication Guide. Ask your doctor if you have any questions about taking AUSTEDO XR.
Download the TD treatment tracking guide to record when
AUSTEDO XR is taken and note any questions for your doctor.
The information on this site is intended for healthcare professionals in the United States. Are you a healthcare professional in the United States?
AUSTEDO® XR (deutetrabenazine) extended-release tablets and AUSTEDO® (deutetrabenazine) tablets are prescription medicines that are used to treat:
It is not known if AUSTEDO XR and AUSTEDO are safe and effective in children.
AUSTEDO XR and AUSTEDO can cause serious side effects in people with Huntington’s disease, including: depression, suicidal thoughts, or suicidal actions. Do not start taking AUSTEDO XR or AUSTEDO if you are depressed (have untreated depression or depression that is not well controlled by medicine) or have suicidal thoughts. Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts or feelings. This is especially important when AUSTEDO XR or AUSTEDO is started and when the dose is changed. Call your healthcare provider right away if you become depressed, have unusual changes in mood or behavior, or have thoughts of suicide.
Do not take AUSTEDO XR or AUSTEDO if you:
Other possible serious side effects include:
Sleepiness (sedation) is a common side effect of AUSTEDO XR and AUSTEDO. While taking AUSTEDO XR or AUSTEDO, do not drive a car or operate dangerous machinery until you know how AUSTEDO XR or AUSTEDO affects you. Drinking alcohol and taking other drugs that may also cause sleepiness while you are taking AUSTEDO XR or AUSTEDO may increase any sleepiness caused by AUSTEDO XR and AUSTEDO.
The most common side effects of AUSTEDO in people with Huntington’s disease include sleepiness (sedation), diarrhea, tiredness, and dry mouth.
The most common side effects of AUSTEDO in people with tardive dyskinesia include inflammation of the nose and throat (nasopharyngitis) and problems sleeping (insomnia).
The most common side effects of AUSTEDO XR are expected to be similar to AUSTEDO in people with Huntington’s disease or tardive dyskinesia.
These are not all the possible side effects of AUSTEDO XR or AUSTEDO. Call your doctor for medical advice about side effects. You are encouraged to report side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please read the accompanying Medication Guide.
Reference:
1. AUSTEDO® XR (deutetrabenazine) extended-release tablets/AUSTEDO® tablets current Prescribing Information. Parsippany, NJ: Teva Neuroscience, Inc.
